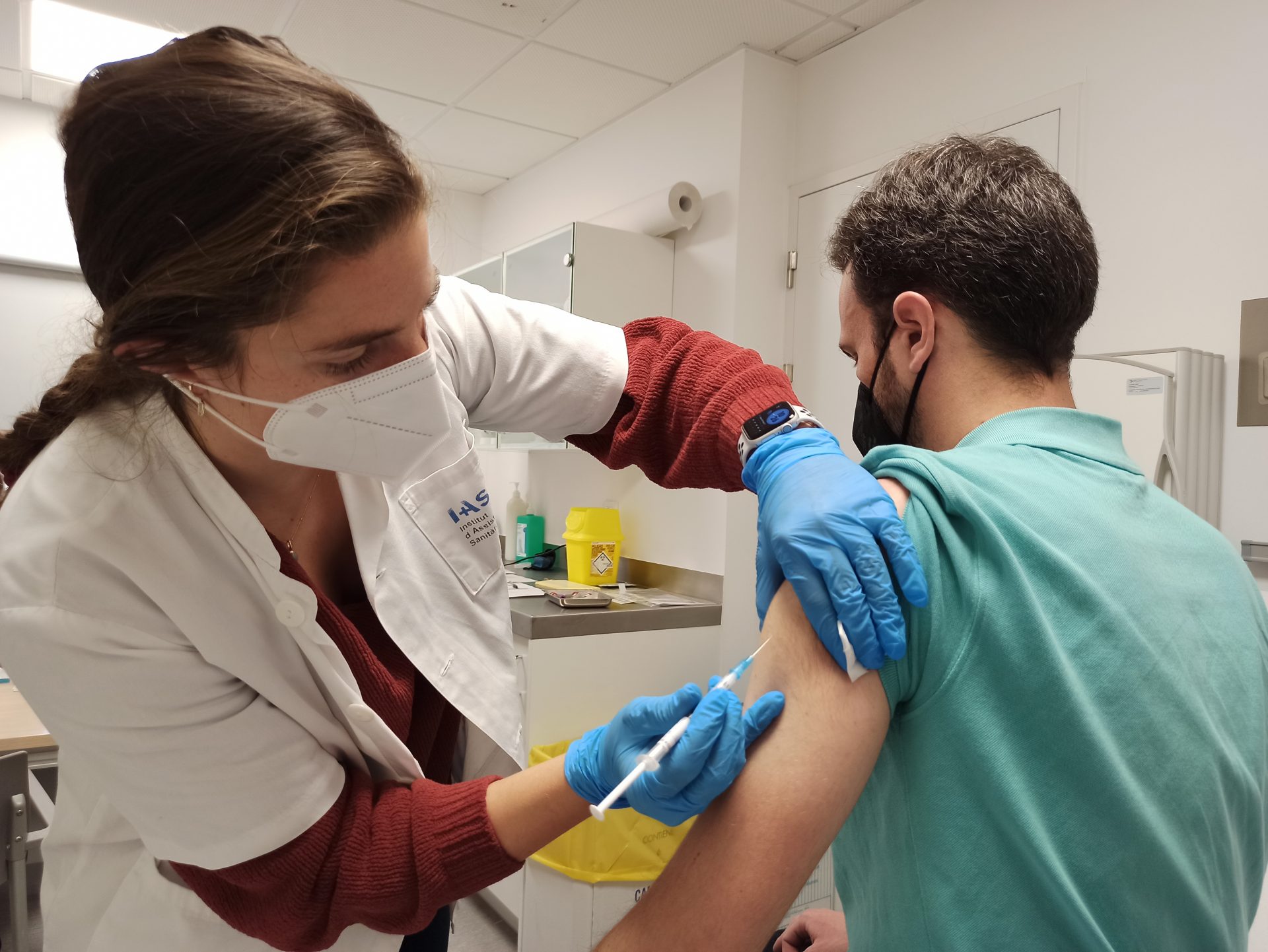
The University Hospital of Girona Dr. Josep Trueta has started administering the Covid-19 vaccine being developed by the HIPRA biotechnology pharmaceutical company to Phase III volunteers this morning. The AEMPS last week authorized the Phase III clinical trial, which begins once Phase IIb has demonstrated good tolerability, a good safety profile and a powerful response to variants, including omicron. This is the last phase of the clinical trial before the vaccine is marketed.
The first volunteers were summoned this morning to Trueta Hospital where, after a questionnaire and clinical tests, they were given the vaccine. In this phase, the Girona center will vaccinate a total of 400 people in two weeks, who will be monitored for one year to assess long-term safety and the immune response.
Phase III, which will involve some 3,000 people over the age of 16 and will take place in 17 hospitals in Spain, 2 in Portugal and 1 in Italy, will continue to evaluate the safety and efficacy of the dose of reinforcement of the HIPRA vaccine against Covid-19 in a larger group of people vaccinated with different vaccines (AstraZeneca, Moderna, Pfizer and Janssen). The aim of this study is to confirm that booster immunization with the HIPRA vaccine may extend protection against new variants and prolong the preventive effect of vaccination.
Participants in the trial must have received at least one or two doses of one of the authorized vaccines (Comirnaty (Pfizer), Spikevax (Moderna), Vaxevria (AstraZeneca), Janssen (Janssen) or a combination) 3 months ago. In the event that they have passed the Covid-19 at least 1 month ago, without having been admitted to the hospital, they will also be able to participate in the study. Each hospital has set up a space on its website for interested people to register.
The HIPRA vaccine
The HIPRA Covid-19 vaccine is an adjuvanted multivariate recombinant protein vaccine based on a receptor-binding domain (RBD) fusion heterodimer containing B.1.1.7 (alpha) and B.1.351 (beta) variants of SARS-CoV-2.
The HIPRA vaccine is stored at refrigerator temperature (between 2 and 8 ºC), which facilitates storage and distribution. However, the technology used allows great versatility to adapt it to new variants of the virus, if necessary in the future. The results obtained to date show that the vaccine produces neutralizing antibodies against current VOCs (variants of "concern") and also effective in the prevention of the disease.
20 hospitals participate in Phase III
Phase III of the HIPRA vaccine involves different public and private hospitals located in Spain (17), Portugal (2) and Italy (1): in Catalonia, in addition to the University Hospital of Girona Dr. Josep Trueta, also involved in the Germans Trias i Pujol Hospital; Hospital Clínic de Barcelona; Vall d'Hebron Hospital Barcelona Hospital Campus Mollet Hospital, HM Nou Delfos Hospital Barcelona and Quironsalud Hospital Barcelona; in addition, the La Paz University Hospital (Madrid) participates in the Community of Madrid; Prince of Asturias University Hospital (Alcalá de Henares); Gregorio Marañón University General Hospital (Madrid); HM Montepríncipe University Hospital (Madrid); HM Sanchinarro University Hospital (Madrid); HM Puerta del Sur University Hospital (Móstoles); Quironsalud University Hospital Madrid (Madrid); in addition to the Cruces University Hospital (Barakaldo, Basque Country); University Regional Hospital of Málaga (Málaga, Andalusia); University Clinical Hospital of Valencia (Valencia, Valencian Community); Hospital Univ. Minho Braga (Braga, Portugal); Algarve Biomedical Center Hospital (Algarve, Portugal); Niguarda Hospital (Milan, Italy).
The clinical trial of the HIPRA vaccine is being conducted by two research teams, with three principal investigators. One of them is the Vascular Health Epidemiology Group of the Institut Català de la Salut in Girona led by Dr. Rafel Ramos -researcher at the University Institute of Primary Care Research (IDIAP) Jordi Gol and the Institute of Biomedical Research of Girona Dr. Josep Trueta (IDIBGI). On the other hand, a multidisciplinary team of medical, nursing and administrative professionals from the Infectious Diseases Service and the Preventive Medicine and Epidemiology Service is working at the Hospital Clínic of Barcelona.