
Cancer is increasingly prevalent in society and the efforts of the research community, doctors and administrations to find solutions to this disease are huge. However, it cannot be treated in a uniform way, as there are more than 200 types of cancer. In addition, the disease in each patient is unique, because the mutations that give rise to the development of each of the tumors are different in each case.
The Girona Biomedical Research Institute Dr. Josep Trueta (IDIBGI) and ICO Girona are part of a European project that seeks to interpret the mutation profile of a specific tumor so that physicians can prescribe the most appropriate treatment for each patient. The project is led by Dr. Núria López-Bigas, ICREA researcher and head of the Biomedical Genomics laboratory at IRB Barcelona. On behalf of IDIBGI, Dr. Ramon Brugada, head of the Cardiovascular Genetics group, and Drs. Joan Brunet and Joaquim Bosch, from the Precision Clinical Oncology research group, participate in the project.
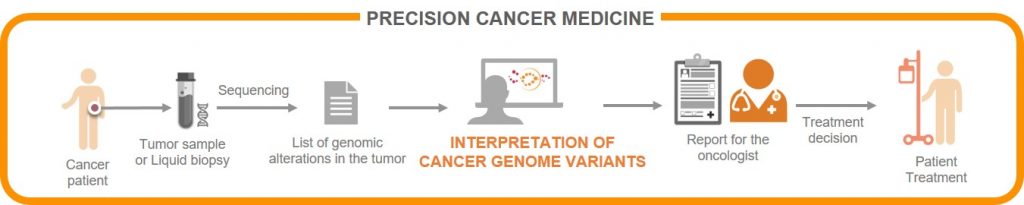
The platform that analyses potential susceptibilities of each tumour is called Cancer Genome Interpreter and it uses machine-learning and other computational methods to systematically extract information from mutations observed in thousands of tumours—28,000 tumours from 66 types of cancer analysed to date—to improve the interpretation of the variants observed in each patient.
“The Cancer Genome Interpreter, which we have been working on for more than five years, has immense potential and, through this project, we intend to optimise it for its use in hospitals and healthcare centres. We want it to be a key instrument to support the decision-making by clinical oncologists, so that each patient, regardless of the hospital in which the diagnosis is made, receives the most appropriate treatment,” explains Dr. López-Bigas.
Spanning five years, the project has been awarded a funding of €10 M by the European Commission, which has greatly valued its main aim: to provide an answer to an unmet medical need. The European Commission has also highlighted the quality of the platform, the expertise of the partners, and the solid implementation plan presented.
"Driver" and "passenger" mutations in cancer
Tumours are characterised by uncontrolled cell growth and proliferation. Along this process, cells accumulate many mutations (in the order of thousands in the entire genome). However, only a few of these (usually fewer than 10) are relevant for the development of the tumour.
The Cancer Genome Interpreter identifies those mutations that are important for cancer development and those that may be related to the response to a given treatment. On these basis, a prediction can be made of whether a specific drug can be effective against that tumour or whether the tumour is likely to be resistant to the treatment. With this information, the system provides a report to help medical doctors to make decisions about treatment options.
The project also includes the set-up of virtual tumor boards integrated by international experts with whom medical doctors can consult and discuss the report produced by the Cancer Genome Interpreter. The purpose of such meetings is to promote the exchange of knowledge and thus offer optimal treatment management to all patients, particularly those who are treated in smaller healthcare centres and who often do not have access to many specialized oncologists.
Patient-centred
A key pillar of this project is the involvement of people with cancer through two associations that will form part of the project, representing society and patients, with multiple goals. First, the aim is to involve patients so that they can play an active role in the process of personalised cancer medicine. To this end, the system also provides patients with a report, thus informing them about molecular features of their tumour.
Second, the project seeks to highlight the value of the information and make patients aware that they can help to improve the system by accepting to share the molecular details of their tumours and their clinical information. In its current version, the Cancer Genome Interpreter has been developed from the analysis of the genomes of the tumours of 28,000 patients, covering more than 60 types of cancer, which are available to the scientific community in public repositories. As new sequenced tumours are added to the public domain, machine-learning methods will improve their predictions, thereby enhancing the interpretation of the tumour mutations for new patients.
The ECPC (the European Cancer Patient Coalition) and the Spanish Association Against Cancer will promote the active participation of cancer patients in this research.
17 partners for an ambitious project
As well as patient associations, IRB Barcelona will be collaborating with 17 different types of European organisations, which offer complementary expertise to address the implementation of personalised cancer medicine from all angles. Nine hospitals and healthcare centres from four European countries are partners of the project and these will be the first to introduce the platform into their systems.
Besides IRB Barcelona, the project partners are: the Girona Biomedical Research Institute Dr. Josep Trueta (IDIBGI); the Vall d’Hebron Institute of Oncology (VHIO); the Gustave Roussy; the Leon Berard Centre de Lutte Contre le Cancer; Uniklinik RWTH AACHEN; Universitätsklinikum Köln AöR; the Manchester Cancer Research Centre; the Fundació Althaia - Hospital Sant Joan de Déu de Manresa and the Andalusian Health Services, as well as the Catalan Institute of Oncology (ICO); the Fundación Progreso y Salud of the Regional Government of Andalusia; the Centro Nacional de Análisis Genómico (CNAG – CRG); the company Alira Health, which is the partner in charge of ensuring regulatory aspects; and the European Association for Cancer Research (EACR), which will be managing communication actions associated with the project.
Crèdits imatge: IRB Barcelona.