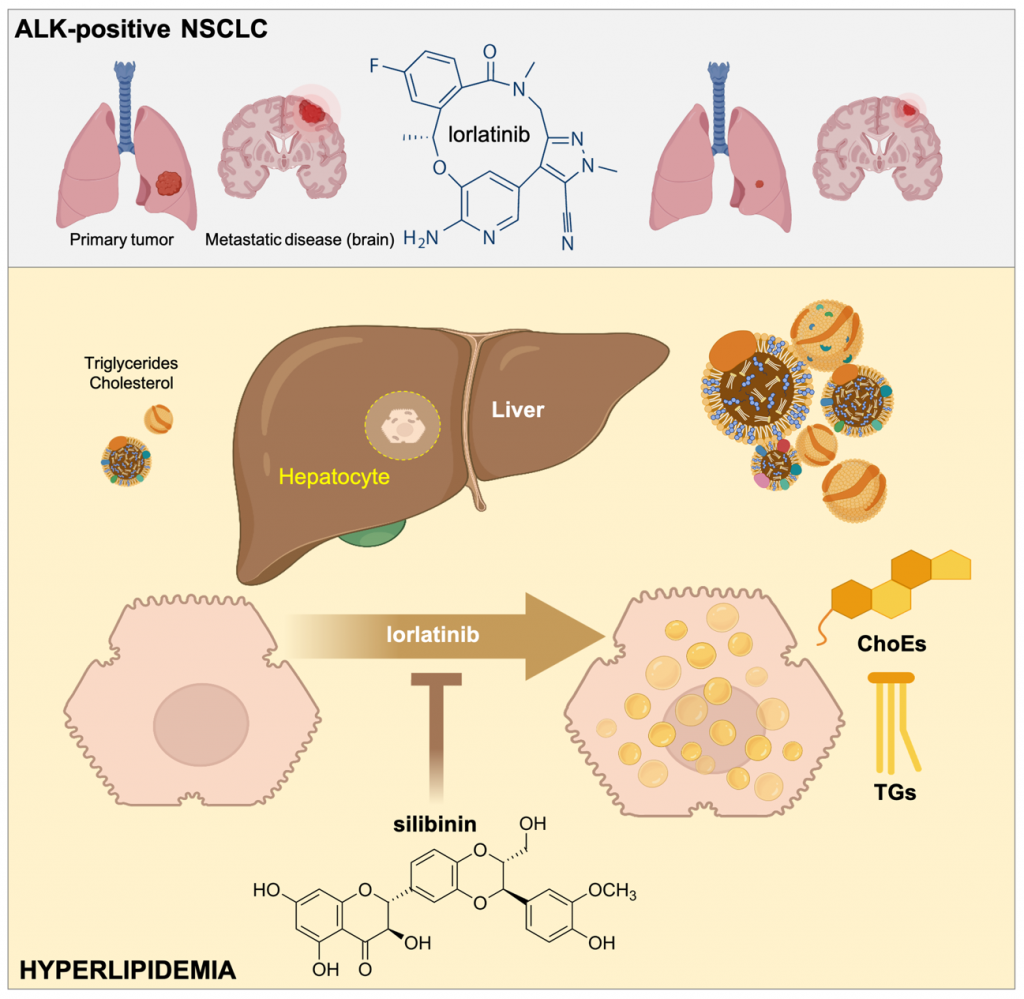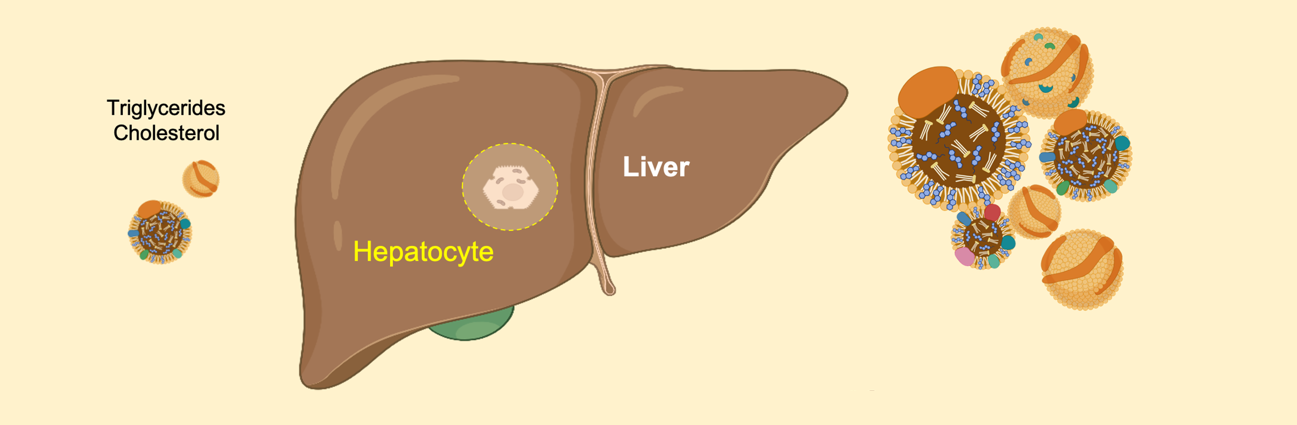
The Metabolism and Cancer group of the ProCURE programme from the Catalan Oncology Institute (ICO) and the Girona Biomedical Research Institute (IDIBGI) has published a study in the International Journal of Molecular Sciences that demonstrates that the elevated levels of cholesterol and triglycerides observed in the plasma of lung cancer patients treated with lorlatinib could come from primary alterations in the hepatic accumulation of numerous types of fats. These results have been obtained in collaboration with the Precision Oncology group in Girona, also from the ICO/IDIBGI, and the Pere Virgili Health Research Institute in Reus.
Using high-resolution tools such as mass spectrometry, the ProCURE-ICO/IDIBGI researchers have discovered that lorlatinib is able to "reprogram" the lipidome –that is, the composition and abundance of lipids– in liver cells, directly promoting the accumulation of numerous different types of cholesterol and triglycerides internally. The researchers have also discovered that silibinin –the bioactive substance in milk thistle with anti-tumor activity– is able to "protect" the lipidome of liver cells, significantly reducing the formation of "fat cells" in the presence of lorlatinib.
Lorlatinib (Lorviqua) is a drug indicated for the treatment of patients with an advanced type of lung cancer who are anaplastic lymphoma kinase (ALK)-positive. The side effects of lorlatinib are different from those seen with other tyrosine kinase inhibitors that block ALK activity. Specifically, those side effects include the occurrence of hypercholesterolemia and hypertriglyceridemia, i.e., a significant increase in blood cholesterol and serum triglyceride levels in up to 80% of patients receiving lorlatinib. This unwanted side effect requires the use of new-generation statins that do not interact with the enzyme responsible for metabolizing lorlatinib: the cytochrome CYP450 3A4. However, the pathophysiological mechanism through which lorlatinib causes these metabolic adverse effects and their possible approach with new therapeutic strategies is unknown.
Link to the study: https://www.mdpi.com/1422-0067/23/17/9986
In collaboration with the Miguel Hernández University and the Institute for Research, Development and Innovation in Health Biotechnology (IDiBE) of Elche, researchers from ProCURE-ICO/IDIBGI have finally evaluated the possible interaction of silibinin with cytochrome CYP450 3A4 to assess the safety profile of a possible combination with lorlatinib in the clinic. By combining "computer simulation" studies and CYP450 3A4 enzyme activity assays, the researchers ruled out possible unwanted interference from silibinin.
The results of the study highlight, for the first time, the ability of lorlatinib to promote aberrant lipid metabolism in human liver cells and that silibinin is a new candidate to clinically control the undesirable hyperlipidemic activity of lorlatinib in ALK-positive lung cancer patients (see Figure).